 https://www.keyscriptsllc.com/wp-content/uploads/2025/06/corestrength.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2025-06-30 18:30:192025-06-30 18:32:38The Role of Core Strength in Rehabilitation
https://www.keyscriptsllc.com/wp-content/uploads/2025/06/corestrength.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2025-06-30 18:30:192025-06-30 18:32:38The Role of Core Strength in Rehabilitation https://www.keyscriptsllc.com/wp-content/uploads/2025/06/corestrength.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2025-06-30 18:30:192025-06-30 18:32:38The Role of Core Strength in Rehabilitation
https://www.keyscriptsllc.com/wp-content/uploads/2025/06/corestrength.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2025-06-30 18:30:192025-06-30 18:32:38The Role of Core Strength in Rehabilitation https://www.keyscriptsllc.com/wp-content/uploads/2024/12/computer.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2025-01-03 18:51:342025-01-06 15:53:09The Power of Technology in Pharmacy Claims Management
https://www.keyscriptsllc.com/wp-content/uploads/2024/12/computer.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2025-01-03 18:51:342025-01-06 15:53:09The Power of Technology in Pharmacy Claims Management https://www.keyscriptsllc.com/wp-content/uploads/2024/12/needle.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-12-05 20:30:292024-12-13 10:27:23Staying Sharp on Needlestick Injuries
https://www.keyscriptsllc.com/wp-content/uploads/2024/12/needle.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-12-05 20:30:292024-12-13 10:27:23Staying Sharp on Needlestick Injuries https://www.keyscriptsllc.com/wp-content/uploads/2024/10/homehealth.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-11-04 08:30:002024-12-05 20:33:25November is National Home Care & Hospice Month
https://www.keyscriptsllc.com/wp-content/uploads/2024/10/homehealth.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-11-04 08:30:002024-12-05 20:33:25November is National Home Care & Hospice Month https://www.keyscriptsllc.com/wp-content/uploads/2024/10/eye.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-10-18 19:26:582024-10-30 16:49:37Insights on Medications Used for Work-Related Eye Injury
https://www.keyscriptsllc.com/wp-content/uploads/2024/10/eye.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-10-18 19:26:582024-10-30 16:49:37Insights on Medications Used for Work-Related Eye Injury https://www.keyscriptsllc.com/wp-content/uploads/2024/09/hands2.png
666
666
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-09-13 19:47:342024-09-16 18:45:50National Suicide Prevention Month Part 2: Risks & Resources
https://www.keyscriptsllc.com/wp-content/uploads/2024/09/hands2.png
666
666
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-09-13 19:47:342024-09-16 18:45:50National Suicide Prevention Month Part 2: Risks & Resources https://www.keyscriptsllc.com/wp-content/uploads/2024/09/hands.png
666
666
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-09-06 20:26:082024-09-13 15:22:34National Suicide Prevention Month Part 1: Facts & Stats
https://www.keyscriptsllc.com/wp-content/uploads/2024/09/hands.png
666
666
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-09-06 20:26:082024-09-13 15:22:34National Suicide Prevention Month Part 1: Facts & Stats https://www.keyscriptsllc.com/wp-content/uploads/2024/08/networkdata_light.png
666
666
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-08-22 19:54:062024-08-26 12:47:35Expanding Pharmacy Network Utilization Through Data Innovation
https://www.keyscriptsllc.com/wp-content/uploads/2024/08/networkdata_light.png
666
666
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-08-22 19:54:062024-08-26 12:47:35Expanding Pharmacy Network Utilization Through Data Innovation https://www.keyscriptsllc.com/wp-content/uploads/2024/07/ffcard.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-07-11 17:47:342024-08-26 12:56:40KeyScripts First Fill Program
https://www.keyscriptsllc.com/wp-content/uploads/2024/07/ffcard.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-07-11 17:47:342024-08-26 12:56:40KeyScripts First Fill Program https://www.keyscriptsllc.com/wp-content/uploads/2024/06/safety.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-06-24 18:34:072024-06-27 08:49:07Keeping Your Workplace Safe
https://www.keyscriptsllc.com/wp-content/uploads/2024/06/safety.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-06-24 18:34:072024-06-27 08:49:07Keeping Your Workplace Safe https://www.keyscriptsllc.com/wp-content/uploads/2024/05/gabapentin.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-05-13 12:22:202024-05-13 13:31:41Brand News: Gralise Goes Generic
https://www.keyscriptsllc.com/wp-content/uploads/2024/05/gabapentin.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-05-13 12:22:202024-05-13 13:31:41Brand News: Gralise Goes Generic https://www.keyscriptsllc.com/wp-content/uploads/2024/04/translation.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-04-18 13:11:412024-04-22 13:49:28KeyScripts Translation Services Network
https://www.keyscriptsllc.com/wp-content/uploads/2024/04/translation.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-04-18 13:11:412024-04-22 13:49:28KeyScripts Translation Services Network https://www.keyscriptsllc.com/wp-content/uploads/2024/03/tbi.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-03-21 17:53:152024-03-29 14:16:49Brain Injury in the Workplace
https://www.keyscriptsllc.com/wp-content/uploads/2024/03/tbi.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-03-21 17:53:152024-03-29 14:16:49Brain Injury in the Workplace https://www.keyscriptsllc.com/wp-content/uploads/2024/02/newrx-red.xls-Shortcut.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-02-22 19:40:512024-02-27 14:32:08TrendWatch: Drug Updates for 2024
https://www.keyscriptsllc.com/wp-content/uploads/2024/02/newrx-red.xls-Shortcut.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-02-22 19:40:512024-02-27 14:32:08TrendWatch: Drug Updates for 2024 https://www.keyscriptsllc.com/wp-content/uploads/2024/01/ds.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-01-29 18:13:572024-01-31 16:39:54Medication Monitoring Services: Drug Screening
https://www.keyscriptsllc.com/wp-content/uploads/2024/01/ds.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2024-01-29 18:13:572024-01-31 16:39:54Medication Monitoring Services: Drug Screening https://www.keyscriptsllc.com/wp-content/uploads/2023/12/medcalendar.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-12-19 16:04:122023-12-26 13:25:35Prescription for Success: Medication Adherence in Workers’ Compensation
https://www.keyscriptsllc.com/wp-content/uploads/2023/12/medcalendar.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-12-19 16:04:122023-12-26 13:25:35Prescription for Success: Medication Adherence in Workers’ Compensation https://www.keyscriptsllc.com/wp-content/uploads/2023/11/rad.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-11-06 09:07:002023-12-26 13:20:00KeyScripts Ancillary Services: Diagnostic Testing
https://www.keyscriptsllc.com/wp-content/uploads/2023/11/rad.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-11-06 09:07:002023-12-26 13:20:00KeyScripts Ancillary Services: Diagnostic Testing https://www.keyscriptsllc.com/wp-content/uploads/2023/10/nptm.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-10-23 13:52:092023-10-23 15:23:14KeyScripts Ancillary Services: Physical Medicine – Part Two
https://www.keyscriptsllc.com/wp-content/uploads/2023/10/nptm.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-10-23 13:52:092023-10-23 15:23:14KeyScripts Ancillary Services: Physical Medicine – Part Two https://www.keyscriptsllc.com/wp-content/uploads/2023/10/pt.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-10-09 13:01:422023-10-16 17:00:43KeyScripts Ancillary Services: Physical Medicine – Part One
https://www.keyscriptsllc.com/wp-content/uploads/2023/10/pt.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-10-09 13:01:422023-10-16 17:00:43KeyScripts Ancillary Services: Physical Medicine – Part One https://www.keyscriptsllc.com/wp-content/uploads/2023/09/cr.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-09-15 19:55:352024-01-31 16:40:50Medication Monitoring Services: What is a Clinical Pharmacy Review?
https://www.keyscriptsllc.com/wp-content/uploads/2023/09/cr.png
2289
2288
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-09-15 19:55:352024-01-31 16:40:50Medication Monitoring Services: What is a Clinical Pharmacy Review? https://www.keyscriptsllc.com/wp-content/uploads/2023/08/target.png
2345
2344
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-08-17 20:08:152023-10-09 13:02:29Migraine Management Part Three: Pharmacologic Therapies
https://www.keyscriptsllc.com/wp-content/uploads/2023/08/target.png
2345
2344
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-08-17 20:08:152023-10-09 13:02:29Migraine Management Part Three: Pharmacologic Therapies https://www.keyscriptsllc.com/wp-content/uploads/2023/07/migraine.png
2345
2344
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-07-26 14:07:302023-10-09 13:02:37Migraine Management Part Two: Nonpharmacologic Therapies
https://www.keyscriptsllc.com/wp-content/uploads/2023/07/migraine.png
2345
2344
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-07-26 14:07:302023-10-09 13:02:37Migraine Management Part Two: Nonpharmacologic Therapies https://www.keyscriptsllc.com/wp-content/uploads/2023/07/sun.png
550
554
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-07-14 14:49:272023-10-09 13:03:52Sun Safety Tips for Employers
https://www.keyscriptsllc.com/wp-content/uploads/2023/07/sun.png
550
554
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-07-14 14:49:272023-10-09 13:03:52Sun Safety Tips for Employers https://www.keyscriptsllc.com/wp-content/uploads/2023/06/brain1.png
2345
2344
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-06-22 14:12:462023-10-09 13:04:00Taking Aim at Migraines: Part One
https://www.keyscriptsllc.com/wp-content/uploads/2023/06/brain1.png
2345
2344
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-06-22 14:12:462023-10-09 13:04:00Taking Aim at Migraines: Part One https://www.keyscriptsllc.com/wp-content/uploads/2023/05/trendwatch-2023.png
767
767
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-05-23 12:21:342023-10-09 13:04:07TrendWatch: Drug Updates for 2023
https://www.keyscriptsllc.com/wp-content/uploads/2023/05/trendwatch-2023.png
767
767
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-05-23 12:21:342023-10-09 13:04:07TrendWatch: Drug Updates for 2023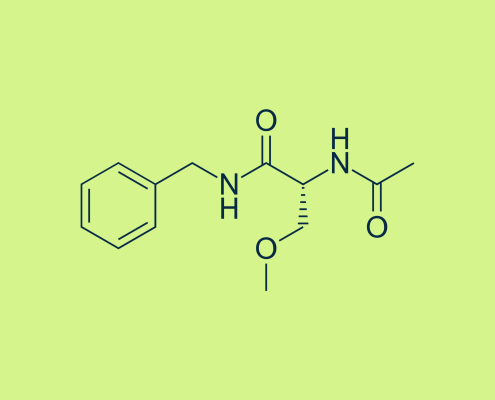 https://www.keyscriptsllc.com/wp-content/uploads/2023/04/lacosamide-molecule.png
2345
2344
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-04-26 15:10:512023-10-09 13:04:14Vimpat Patent Expiration and the Impact of Lacosamide
https://www.keyscriptsllc.com/wp-content/uploads/2023/04/lacosamide-molecule.png
2345
2344
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-04-26 15:10:512023-10-09 13:04:14Vimpat Patent Expiration and the Impact of Lacosamide https://www.keyscriptsllc.com/wp-content/uploads/2023/03/crutches.png
2345
2344
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-03-28 12:35:562023-10-09 13:04:22The ABCs of DME
https://www.keyscriptsllc.com/wp-content/uploads/2023/03/crutches.png
2345
2344
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-03-28 12:35:562023-10-09 13:04:22The ABCs of DME https://www.keyscriptsllc.com/wp-content/uploads/2023/02/arrows.png
2345
2344
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-02-27 17:11:062023-10-09 13:04:30The Power of Prior Authorization
https://www.keyscriptsllc.com/wp-content/uploads/2023/02/arrows.png
2345
2344
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-02-27 17:11:062023-10-09 13:04:30The Power of Prior Authorization https://www.keyscriptsllc.com/wp-content/uploads/2023/01/specialty.png
2350
2349
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-01-24 18:18:472023-10-09 13:04:38What’s So Special About Specialty Drugs?
https://www.keyscriptsllc.com/wp-content/uploads/2023/01/specialty.png
2350
2349
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2023-01-24 18:18:472023-10-09 13:04:38What’s So Special About Specialty Drugs? https://www.keyscriptsllc.com/wp-content/uploads/2022/11/pills.png
682
682
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2022-11-30 16:40:572023-10-09 13:04:46Pain Management Part Two: Pharmacologic Treatment
https://www.keyscriptsllc.com/wp-content/uploads/2022/11/pills.png
682
682
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2022-11-30 16:40:572023-10-09 13:04:46Pain Management Part Two: Pharmacologic Treatment https://www.keyscriptsllc.com/wp-content/uploads/2022/09/exercise.png
1815
1815
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2022-09-20 19:48:212023-10-09 13:04:53Pain Management Part One: Non-Pharmacologic Treatment
https://www.keyscriptsllc.com/wp-content/uploads/2022/09/exercise.png
1815
1815
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2022-09-20 19:48:212023-10-09 13:04:53Pain Management Part One: Non-Pharmacologic Treatment https://www.keyscriptsllc.com/wp-content/uploads/2022/08/brain.png
1815
1815
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2022-08-15 13:07:292023-10-09 13:05:01Opioid Use Disorder
https://www.keyscriptsllc.com/wp-content/uploads/2022/08/brain.png
1815
1815
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2022-08-15 13:07:292023-10-09 13:05:01Opioid Use Disorder https://www.keyscriptsllc.com/wp-content/uploads/2022/06/heartbeat.png
1815
1815
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2022-06-28 14:30:232023-10-09 13:05:09KeyPoints: Part Two of Our Naloxone Series
https://www.keyscriptsllc.com/wp-content/uploads/2022/06/heartbeat.png
1815
1815
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2022-06-28 14:30:232023-10-09 13:05:09KeyPoints: Part Two of Our Naloxone Series https://www.keyscriptsllc.com/wp-content/uploads/2021/11/iw-vector.png
492
492
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2021-11-10 20:36:102023-10-09 13:05:22KeyPoints: Amid the Opioid Epidemic, Reversal Agents Save Lives
https://www.keyscriptsllc.com/wp-content/uploads/2021/11/iw-vector.png
492
492
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2021-11-10 20:36:102023-10-09 13:05:22KeyPoints: Amid the Opioid Epidemic, Reversal Agents Save Lives https://www.keyscriptsllc.com/wp-content/uploads/2021/07/creams-and-solutions-square.png
592
592
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2021-07-01 11:07:282023-10-09 13:05:31KeyPoints: With Diclofenac, Concentration Is Key
https://www.keyscriptsllc.com/wp-content/uploads/2021/07/creams-and-solutions-square.png
592
592
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2021-07-01 11:07:282023-10-09 13:05:31KeyPoints: With Diclofenac, Concentration Is Key https://www.keyscriptsllc.com/wp-content/uploads/2021/02/ks-quotes-square.png
684
692
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2021-02-15 10:24:112023-10-09 13:05:39Brand News: Truvada Patent Expiration Already Paying Dividends
https://www.keyscriptsllc.com/wp-content/uploads/2021/02/ks-quotes-square.png
684
692
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2021-02-15 10:24:112023-10-09 13:05:39Brand News: Truvada Patent Expiration Already Paying Dividends https://www.keyscriptsllc.com/wp-content/uploads/2020/11/piggy-bank-purple.png
488
488
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2020-11-02 08:59:392023-10-09 13:05:47Generically Speaking… Part Three of Our Three-Part Series
https://www.keyscriptsllc.com/wp-content/uploads/2020/11/piggy-bank-purple.png
488
488
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2020-11-02 08:59:392023-10-09 13:05:47Generically Speaking… Part Three of Our Three-Part Series https://www.keyscriptsllc.com/wp-content/uploads/2020/10/tab-and-cap-orange.png
608
608
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2020-10-09 14:20:182023-10-09 13:05:59Generically Speaking… Part Two of Our Three-Part Series
https://www.keyscriptsllc.com/wp-content/uploads/2020/10/tab-and-cap-orange.png
608
608
ksAdvancedMedia
https://www.keyscriptsllc.com/wp-content/uploads/2025/02/KeyScripts-Logo-2025.png
ksAdvancedMedia2020-10-09 14:20:182023-10-09 13:05:59Generically Speaking… Part Two of Our Three-Part Series
CONTACT US
Corporate Center:
1970 Technology Parkway
Mechanicsburg, PA 17050
Phone: 1-866-446-2848
Fax: 717-732-9467
Check out our latest blog
- The Role of Core Strength in Rehabilitation June 30, 2025

